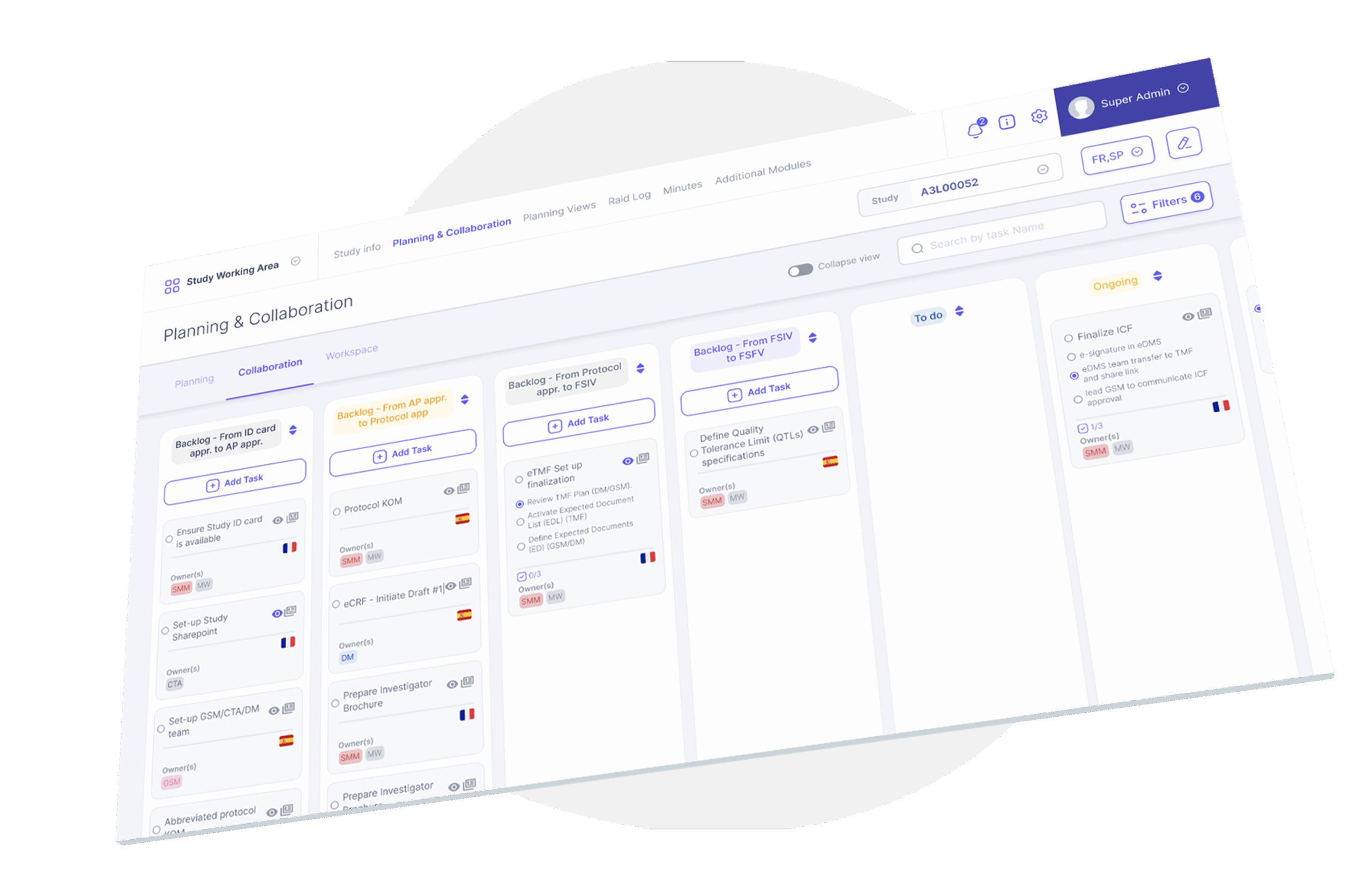
Study Cockpit: added value for your Veeva CTMS infrastructure
Veeva CTMS provides a solid foundation for clinical trial management that is trusted by life sciences organisations, offering compliance, integration capabilities, and continuous innovation.